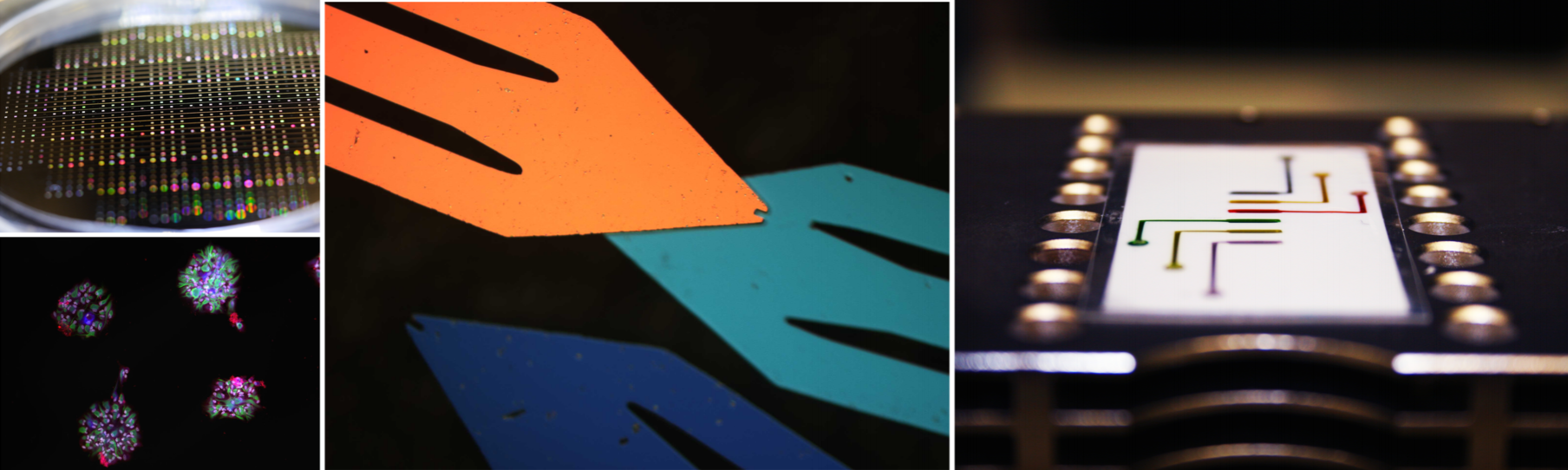
Research Projects
Current Projects
Capillarics
Single Exosome Detection
Circulating Tumour Cells
Gastrointestinal Tract-in-a-Box
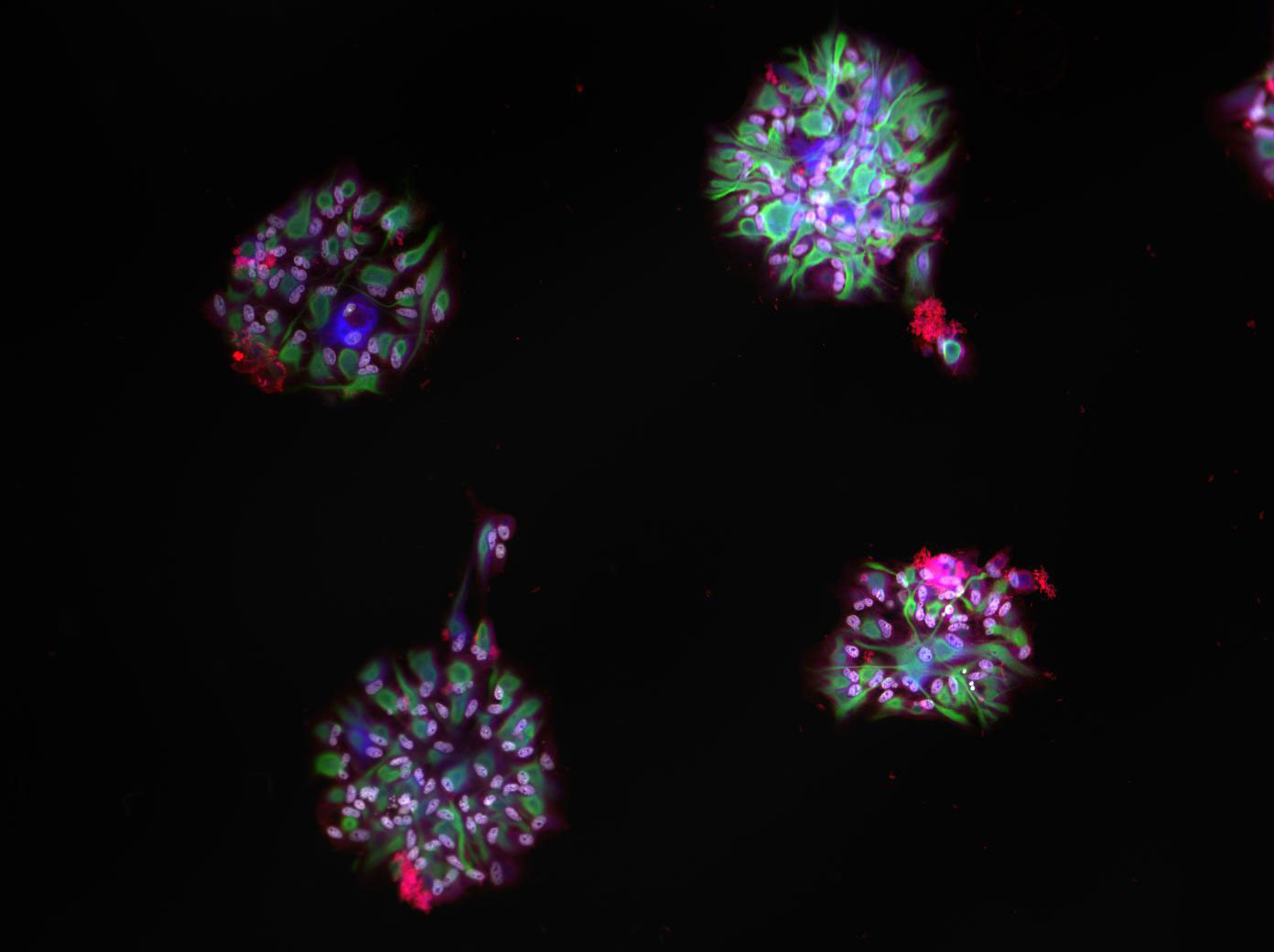
Microscale Cell Co-Culture Patterning
3D Bioprinting
Spatial Secretomics
Past Projects
Reproducibility in Science
Antibody Colocalization Microarray
Soft Neural Implants
Global Health & Environmental Monitoring
Synthetic Biology
Microfluidic Probe
Micro & Nanoscale Protein Patterning
We developed a library of microfluidic capillary elements consisting of different capillary pumps, valves and fluidic resistors. We propose to use this toolbox to make various autonomous capillary systems and present some of their capabilities, which have never been investigated. One of the highly interesting platforms is a point-of-care device for methicillin-resistant Staphylococcus aureus (MRSA) detection in high-risk patients in hospitals. Current methods for diagnosis of bacterial infections, such as real time PCR and culturing methods are slow and limited in the number of tests which can be run and the length of time required. Using capillaric elements, we are developing a self-powered microfluidic platform that is simple to operate, compatible with common clinic laboratories and can be used at the point-of-care to deliver results within hours and allow hospital staff to isolate patients with resistant infections to contain their spread.
3D-printed Capillary Microfluidic Circuits for Pre-Programmed Liquid Delivery
Our lab recently developed capillaric circuits (CCs) – advanced capillary microfluidic devices assembled from modular capillary fluidic elements similar to the design of electric circuits (Safavieh & Juncker, Lab Chip, 2013, 13, 4180–4189). CCs choreograph liquid delivery operations according to pre-programmed capillary pressure differences with minimal user intervention. We now manufacture CCs rapidly and inexpensively using 3D-printing technology (Olanrewaju et al, Lab Chip, 2016, 16, 3804–3814). We established design rules for CCs using a combination of analytical modelling and experimentation. Using an electrical circuit analogue of the CC, we established design rules to ensure strictly sequential liquid delivery and demonstrate CCs that autonomously delivered eight liquids in a pre-determined sequence in <7 min. Ongoing work is focused on applying CCs in point-of-care diagnostic applications as described below:
Capillaric circuits for ultra-rapid and user-friendly detection of urinary tract infection
Up to 50% of women will get a urinary tract infection (UTI) by age 32.1 Yet, conventional bacteria detection to identify E. coli – the cause of more than 80% of UTI cases – is slow, centralized, and labour-intensive. Microfluidic devices for bacteria detection often provide high sensitivity but at the cost of increased complexity and instrumentation. To address this challenge, we developed an automated sandwich immunoassay in CCs for sensitive bacteria detection with minimal sample preparation and limited user intervention. We use large‑volume CCs for direct detection of E.coli cells in clinically‑relevant sample volumes (100 µL) in <10 min. Assays were performed in a “walk away” format where the user pre‑loads the sample and reagents into reservoirs, and the capillaric circuit determines the timing and sequence of liquid delivery without further user intervention. Ongoing work is focused on validating the CC using clinical samples and integrating a hand-held reader for rapid and inexpensive detection.
Capillary microfluidic circuits to quantify blood antibody concentrations for vaccine efficacy studies
Measles is a highly contagious, acute viral infection that is transmitted through air, with symptoms including high fever, cough, inflamed eyes and an entire body rash. In 2015, 2.5 million people were infected with measles worldwide, with roughly 10% of the outbreaks occurring in developed countries where measles vaccination program reaches 95% efficacy (data taken from the World Health Organization).Frequent outbreaks of measles have elevated concerns regarding individual’s immunization status and their effective vaccine-mediated immunity against the disease. Frequent outbreaks in North America (139 cases in 2015) show evidence that in adults, Conventional clinical test are reliable, however these tests require large sample and reagent volumes, are slow to administer, and hence inadequate in the event of a breakout. In effect, the need for and the lack of rapid tests to evaluate the immunization status of individuals at risk of a breakout is apparent. In this project, we are developing a alternative immunoassay using capillaric circuits (CC) device fabricated via 3D-pinting, that is capable of detecting anti-measles specific antibodies in 6 μl of spiked sample within 6 minutes. This platform holds promise to be developed into a portable platform for rapid antibody titer measurements.
Personalized treatment for cancer has the potential to adapt to the multiplicity of cancer, to increase the efficacy of drugs and decrease their toxicity. As part of this strategy, liquid biopsy explores a new non-invasive approach to diagnose cancer, guide treatment and monitor efficacy through biomarkers such as tumor derived extracellular vesicles or exosomes.
Exosomes are nanometric membranes vesicles (50 to 150nm in diameter) present in blood and other bodily fluids, playing a central role of mediation in cell-to-cell communication by extravesicular ligands activation and by transfer of molecules between cells. Exosomes are promising biomarkers for cancer with high potential to complete current analysis and help to decrease limits of cancer detection, resulting in earlier and more accurate diagnostic and treatment.
Leveraging the group’s expertise in micro and nano-scale surface patterning, we are developing new methods and tools to capture and detect down to single exosomes. Our research focuses on characterizing exosome heterogeneity in terms of protein, DNA, and RNA content with the aim to further understand exosome signaling.
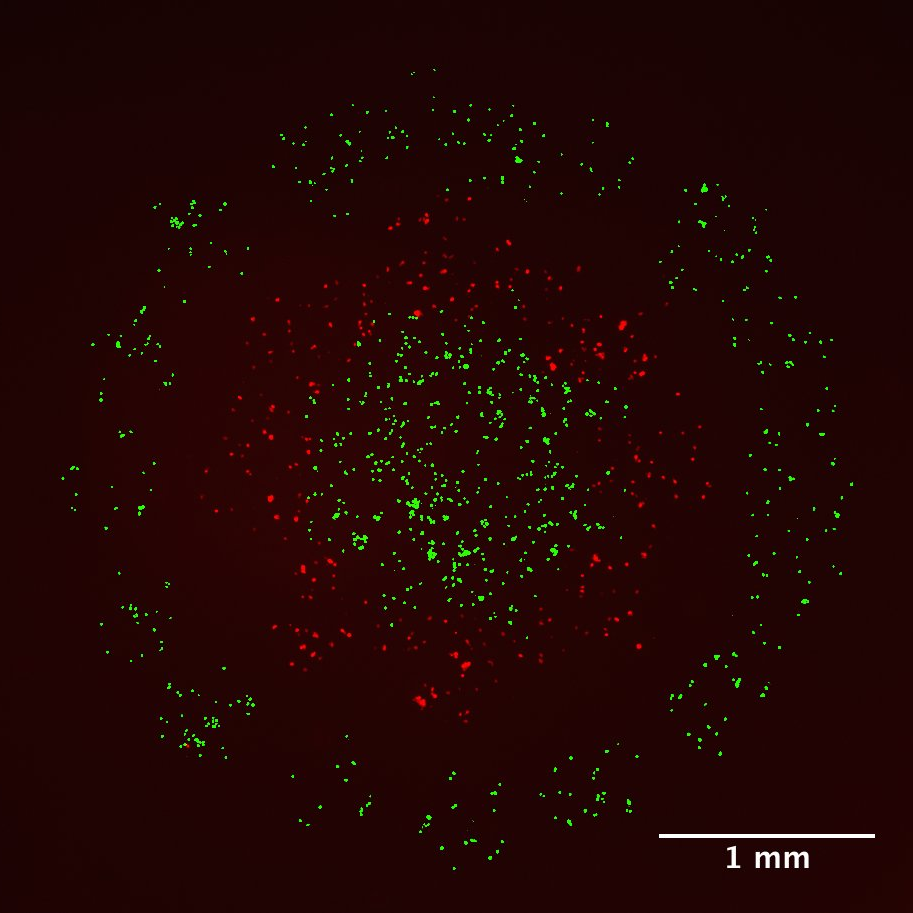
Circulating tumour cells (CTCs) are recognized as a powerful indicator for cancer prognosis. As the disease progresses, cancer cells are shed from tumours into the bloodstream, where they may undergo physiological, chemical, and genetic changes before colonizing distant tissues for metastasis. In collaboration with Dr. T. Veres (National Research Council of Canada), and using replication techniques, we have developed polymer filters with various thicknesses, porosities or pore sizes for CTC capture from blood. Filters are inserted in a 3D printed filtration cartridge, designed to fit with the filter dimensions. After systematic optimization of the filtration process, it has been shown that CTC capture efficiency is significantly enhanced using filters functionalized with antibodies specific to the target cells (mechanical and molecular capture) relative to pristine filters only (mechanical capture only). A multistage filtration cartridge and filters functionalized with various or cocktails of antibody would allow further improving capture performances or targeting CTC subpopulations.
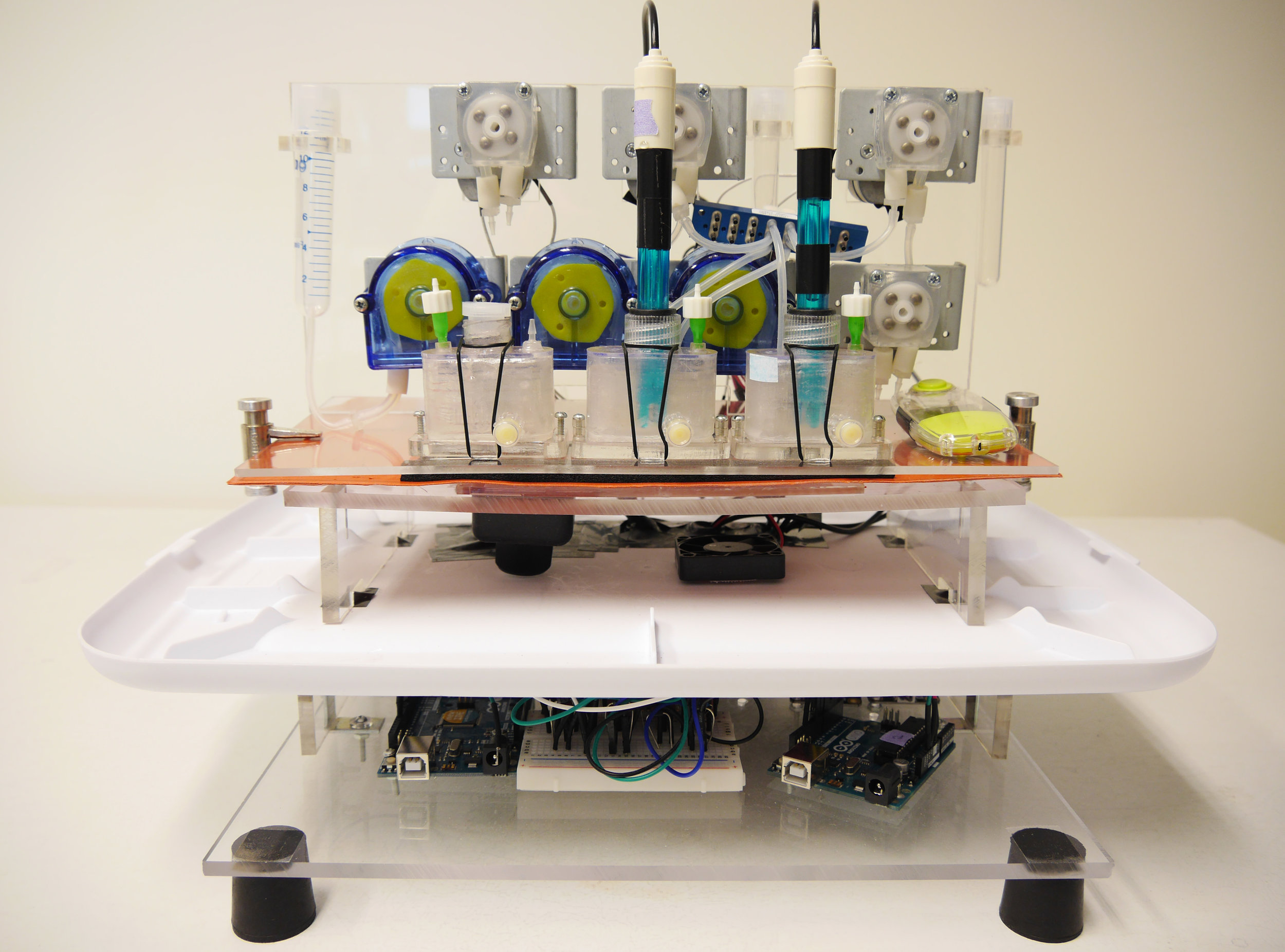
The composition of the human gut microbiome is linked to many diseases and disorders. To better understand this relationship, current technology has focused on human fecal sample analysis, usage of gnotobiotic mice, offering limited controllability and in the latter case, questionable translatability to human. Current in vitro simulation of gut microbiota colonization and development however have been limited by the complexity, scalability, and low throughput. Therefore we propose an in vitro artificial gut system (GITBox) that overcomes the key limitations of previous systems through a miniaturized, modular and multiplex approach with high throughput, aiming at a wide range of biological experimentation such as long-term fecal culture and characterization of microbial compositions relating to different gut physiological conditions.
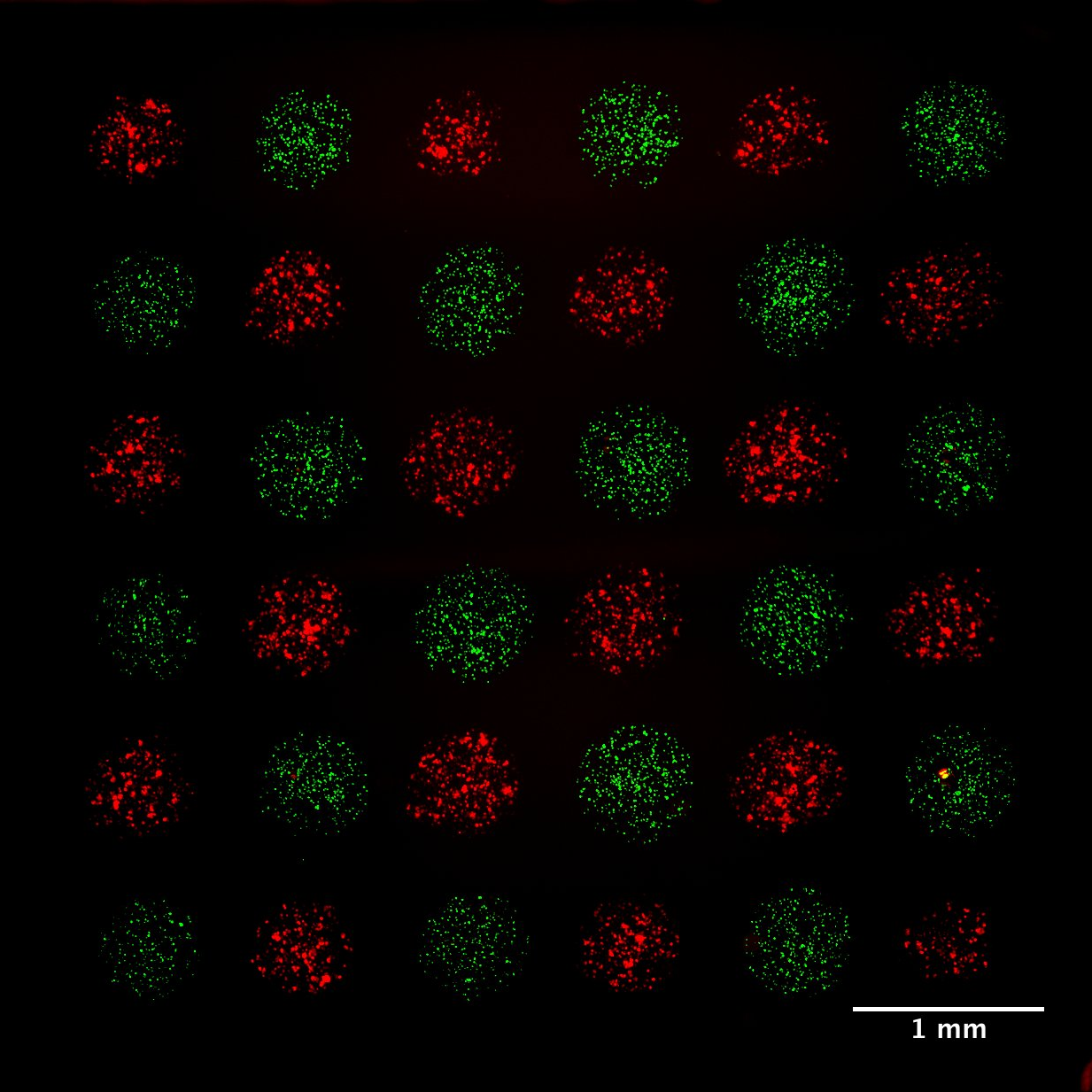
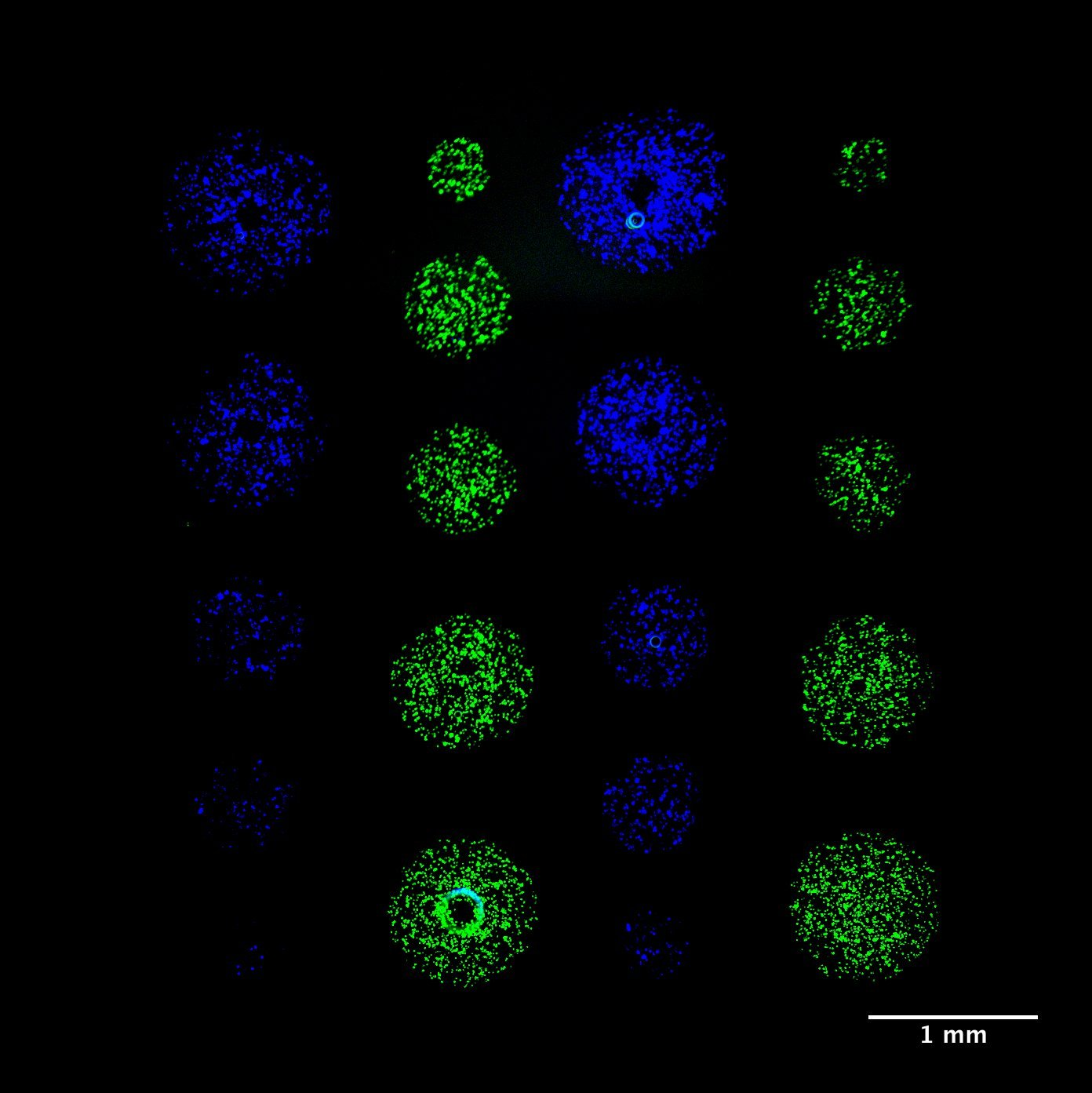
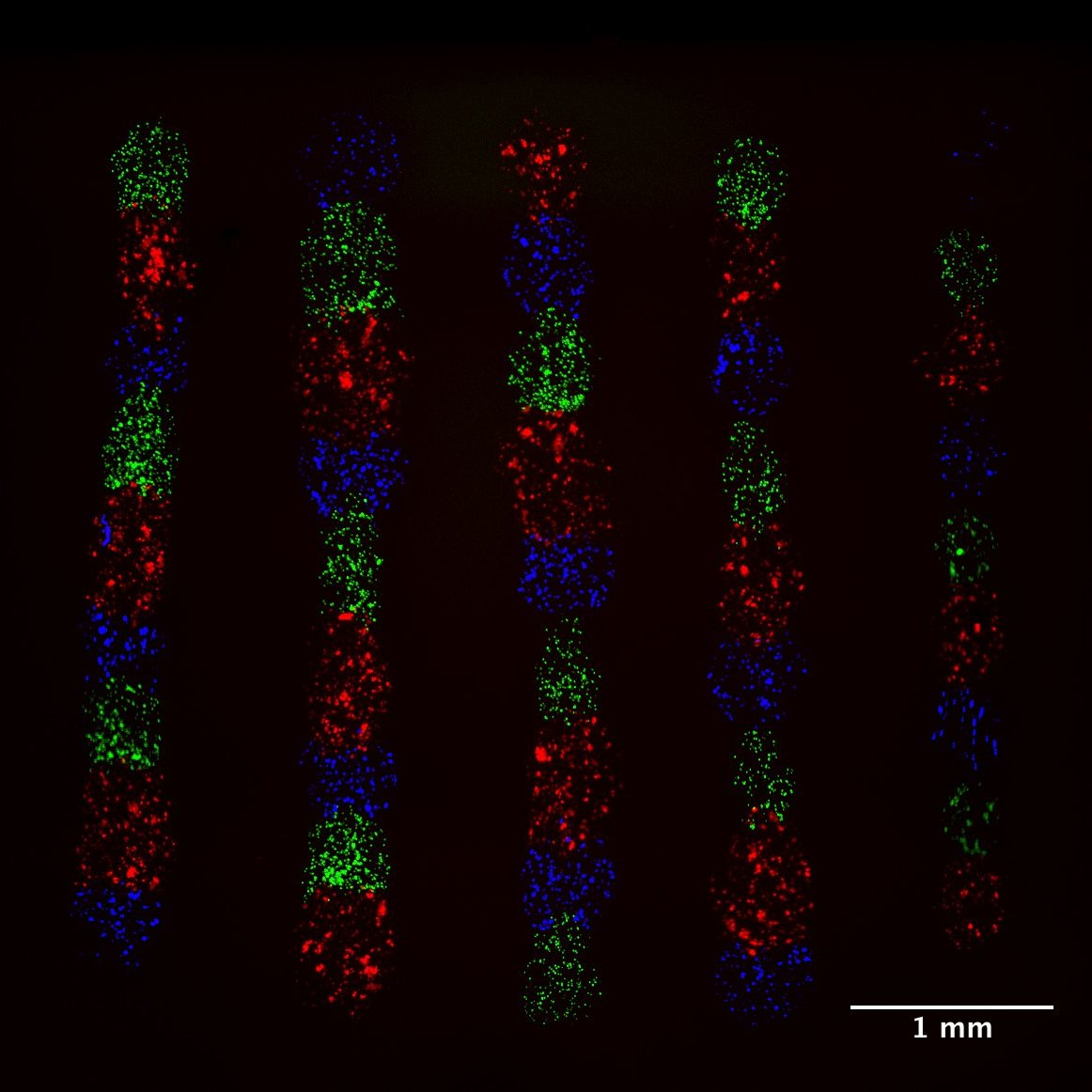
Micropatterning is empowering research for applications in DNA, protein and cell studies. Cell signalling in direct contact. Microscale for high throughput experiments. To study cell interactions and prevent evaporation and provide biocompatible environments, bioinks often contain additives or polymers – for example, hydrogels or aqueous two-phase systems (ATPS) – that can yield viscous solutions. However, typical inkjet or pin-spotters cannot accommodate highly viscous inks, and patterning is done on a larger, macroscopic scale either manually or by extrusion-based systems Here we introduce novel, 3D-printed monolithic pinheads (MPHs) for aligned micropatterning of 100-500 μm spots (~1-250 nl) of highly viscous bioinks containing cells or proteins. Precise alignment is achieved using MPHs with pyramidal bases that fit tightly into well-gaskets that guide and position pins toward the surface for both inking and spotting. A range of different bioinks of variable viscosity can be patterned by serially inking and spotting them with multiple, custom designed 3D-printed MPHs.
Recent microfabrication, microfluidics, and tissue engineering advances have enabled the development of “organ-on-a-chip” models that recapitulate relevant features of human physiology in vitro. Organ-on-a-chip engineering could revolutionize new compound screening and biomarker discovery. However, current platforms only reproduce a very limited set of organs which lack many critical organ functions such as vasculature that limits their potential to a great extent. Stereolithography-based 3D printing has emerged as a high-throughput additive manufacturing method offering sufficient biocompatibility for cell seeding and encapsulation. Creating organs-on-a-chip models with integrated porous barriers using an automated 3D bioprinting process could reduce the design and processing time, avoid assembly and bonding complications, and shorten manufacturing costs compared to conventional processes. In spite of advantages mentioned above, several challenges for bioprinting of biomimetic vascularized tissue constructs still remain, including incapability for continuous printing of hydrogels with clinically relevant dimensions and the inability to fabricate multimaterial complex constructs with high accuracy.
we are currently developing a novel multimaterial 3D bioprinting technology to automatically and rapidly print polymer scaffolds, with controlled micrometer porosity and an embedded perfusable 3D vasculature.
PAST PROJECTS
Selected Publications
K.F.A. Clancy, S. Dery, V. Laforte, P. Shetty, D. Juncker, D.V. Nicolau, Protein microarray spots are modulated by patterning method, surface chemistry and processing conditions, Biosensors and Bioelectronics, 130, 397-407 (2019). PDF | SI
D. Juncker, S. Bergeron, V. Laforte, and H. Li. Cross-Reactivity in Antibody Microarrays and Multiplexed Sandwich Assays: Shedding Light on the Dark Side of Multiplexing. Current Opinion In Chemical Biology, 18, 29-37 (2014). PDF
K.F.A. Clancy, S. Dery, V. Laforte, P. Shetty, D. Juncker, D.V. Nicolau, Protein microarray spots are modulated by patterning method, surface chemistry and processing conditions, Biosensors and Bioelectronics, 130, 397-407 (2019). PDF | SI
We have developed a microarrayer system for making a multiplex assay free of liability pairs which lead to false positives in current sandwich immunoassays. We are utilizing this platform for profiling the proteins in complex biological samples with high sensitivity, high selectivity and high reliability. We are applying this platform to the study of patient samples in many areas, notably breast cancer and traumatic brain injury in collaboration with clinicians and researchers in oncology (for more information, please see the separate page on our Breast Cancer Research). Recently, we have developed a handheld version of the platform called the Snap Chip that can collectively transfer antibody droplets from microarray to microarray upon storage of pre-spotted slides, thus avoiding the use of a microarray spotter to perform colocalization immunoassays.
Brain implants have significant implications in treating neurological disorders and diseases and are a key enabler of brain machine interface (BMI) technology. However, brain implants’ widespread adoption in clinical settings is undermined by challenges in obtaining high-quality recordings and issues of long-term reliability caused by the elicited brain foreign body response (FBR). The brain FBR is exacerbated by the substantial mismatch in Young’s modulus (E) between current implants made from materials like silicon (150 - 200 GPa) and brain tissue (0.4 - 15 kPa). Recent research directed towards improving the mechanical compatibility of brain implants has given rise to more compliant implants made from materials like polyimide (4 - 8 GPa) that are flexible but are still orders of magnitude stiffer than the brain.
We have developed a novel fabrication and implantation method for the softest sub-millimetre intracortical implant to date made from Ecoflex, a silicone elastomer with E = 20 kPa. These soft implants with brain-like stiffness have been shown to reduce the brain FBR compared to both stiffer implants suggesting that soft implants made from materials closer to brain stiffness such as Ecoflex could potentially demonstrate superior high-quality recordings and long-term reliability by reducing the brain FBR.
Infectious diseases are among the most critical health problems for people living in both the developed and developing world. Emerging pathogens, from viral infections such as HIV/AIDS, SARS, and avian influenza, to bacterial infections such as Cholera and Tuberculosis (TB) are a constant and rapidly evolving threat. We seek to address the need for rapid, low-cost diagnostics by building tools for disease testing outside of the hospital or research lab setting. This requires the use of novel materials and systems which may autonomously generate flow or heat, for example. Leveraging our expertise on affinity binders (e.g., antibodies and aptamers), assay development and nano/microengineering, we are developing new technologies and tools that are low-cost, rapid, easy-to-use and reliable, to address challenges in global health and environment.
Thread-based assays
An emerging project involves the use of common cotton threads with their capillary-driven wicking properties to carry out immunoassays. The assay results become visible to the eye within a few minutes after the sample application. We have established binding curves for C-reactive protein (CRP) in buffer and diluted serum and a limit of detection of 377 pM was obtained. We have also demonstrated the possibility of multiplexing using three knotted threads coated with antibodies against CRP, Osteopontin, and Leptin proteins. The results suggest that thread is a suitable support for making low-cost, sensitive, simple-to-use, and multiplexed diagnostic tests. This research has lead to several publications, i.e. an article in Lab on a Chip, featured in research highlights; an article in Analytical Chemistry.
Electricity and Instrument-free Thermocycler
We are developing a low cost thermal-cycler capable of performing polymerase chain reaction (PCR) in low resource settings. Using chemical heating as an energy source and multiple phase change materials for temperature control, PCR can be achieved completely electricity free.
Heavy metal detection kit for drinking water
Funded by IC-IMPACTS, and in collaboration with Dr. Rohit Srivastava at IIT-Bombay, we are developing a low-cost heavy metal drinking water test that generates a colorimetric Safe/Unsafe signal based on an aptamer-based sensing assay. The test does not require complex transport and storage, and allows deployment at low-resource settings. Prototypes will be tested on-site in rural areas in Canada and villages in India.
Analyte enrichment and sensing system for water pollutants
Funded by NSERC Strategic Project Grants, we are extending our work on bead-based assays, capillaric circuits and aptamer-based small molecule binding assays, to develop an aptamer-based analyte enrichment and detection system. In this project we embark on a new collaboration with Dr. Mohamed Siaj at Université du Québec à Montréal to develop a graphene sensor chip that monitors analyte binding in duplexed aptamers, to quantitatively measure the amount of pollutants in environmental water samples.
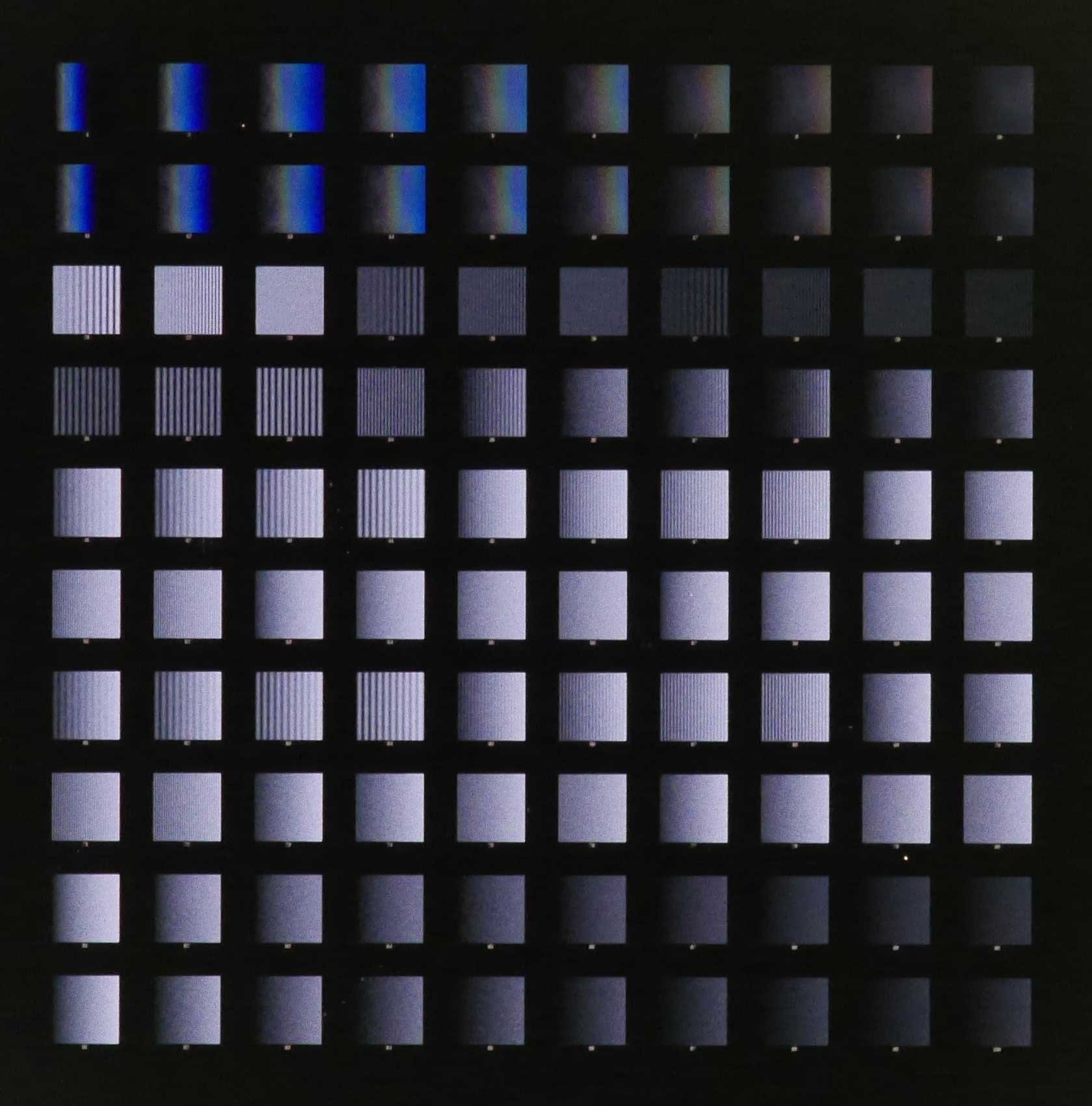
To better investigate the role of concentration gradients of proteins in the guidance of neurons in development, much work has been conducted to replicate these gradients in vitro but has thus far produced tools that are insufficient to properly address in vivo gradients. Since most in vivo gradients are substrate-bound, there is a significant drive to devise a method to replicate such gradients in vitro to better study and understand their functioning. We successfully developed a rapid, low-cost, high throughput nanocontact lift-off process which facilitates the patterning of protein features down to 100 nm. We then introduced novel algorithms to form ordered/randomn and monotonic/non-monotonic digital nanodot gradients (DNGs) with a dynamic range of 3.85 orders of magnitude, exceeding previous reports by almost 2 orders of magnitude. The generation of such patterns will allow us to investigate the role of major guidance proteins with applications in nervous system regeneration. Currently, an extension of this research involves incorporating these guidance cues into microelectrode arrays and neural probes, with aims to improve the cell-electrode interface on these devices and increase the signal quality.
Nucleic acid aptamers, single stranded DNA or RNA sequences that bind a ligand with high affinity and specificity, are commonly deployed in biosensing applications in synthetic biology. The duplexed aptamer (also referred to as a structure-switching aptamer) is one of the most common aptamer biosensor formats. A duplexed aptamer is engineered by hybridizing an aptamer sequence with an aptamer-complementary element (ACE, such as a short DNA oligonucleotide) to form a synthetic ligand-responsive switch. We are leveraging our expertise in microarrays and assay development to study the functional basis of duplexed aptamer-ligand interactions. In this direction, we recently demonstrated that ACEs regulate ligand binding in ATP-specific duplexed aptamers in a non-obvious manner, a finding that also applies to other DNA and RNA aptamers, as well as riboswitches found in nature. This research is opening a new avenue to explore as we seek to understand and better design ligand-responsive nucleic acid biosensors.
Single cell de novo DNA analysis relying on Next-Generation Sequencing is being used to map the heterogeneity of cancerous neoplasms and circulating cells, which informs diagnosis and suggests an improvement to treatment outcomes. However, current single cell DNA analysis methods suffer from (i) bias from the need for PCR amplification which preferentially amplifies certain sequences and (ii) limited ability to sequence repetitive elements, resulting in (iii) an inability to obtain information of long range genomic features.
We are developing a single cell DNA characterization technology for coarse grain DNA mapping which circumvents these limitations using “denaturation mapping”, a single molecule fluorescence-based mapping technique. This is combined with DNA molecule linearization using microfluidic chips in order to detect long-range features on the chromosomes of single cells. Furthermore, we are expanding our protocol to the bench-top through the use of alginate microparticle (AMP) encapsulation for single cell handling and lysis, and thereby reduce the need for on-chip complexity.
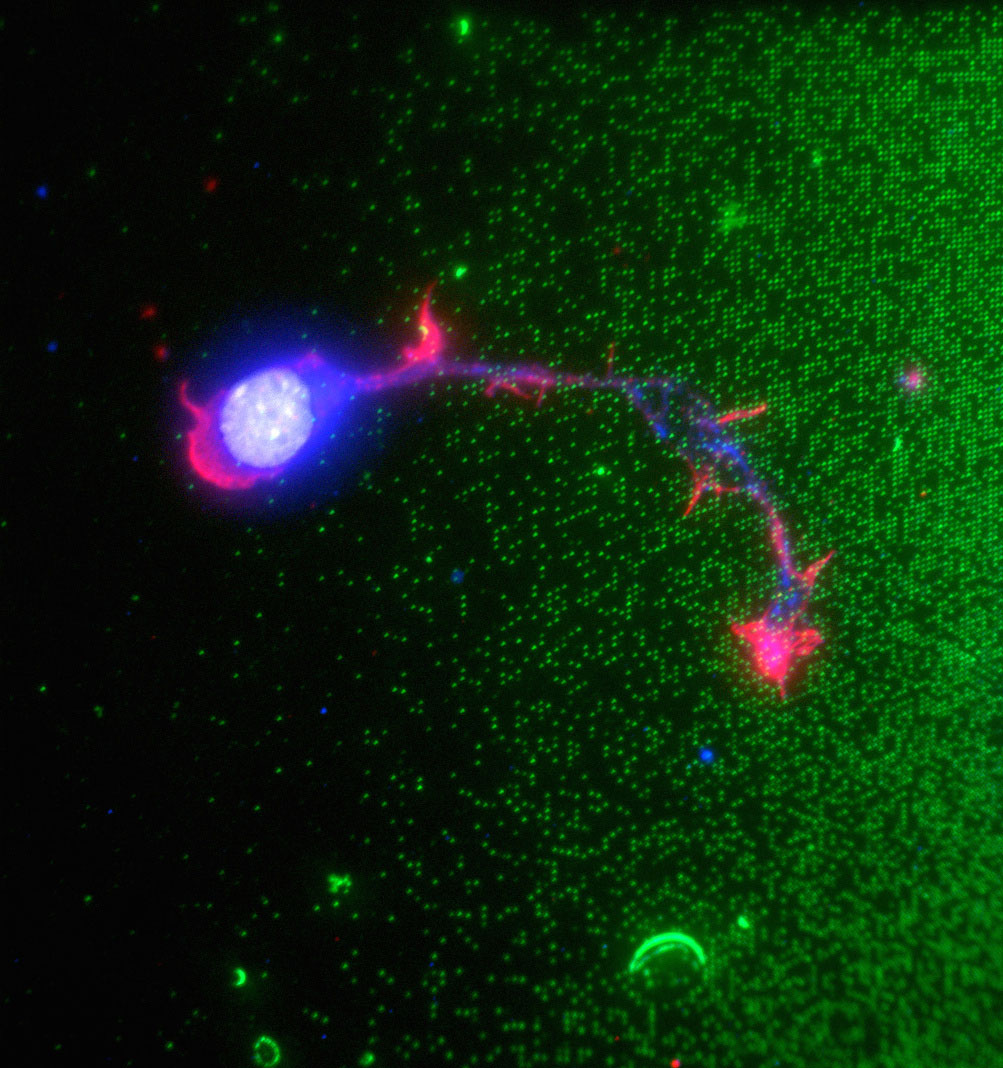
We have developed a microfluidic probe (MFP) that combines the concepts of microfluidics and of scanning probes and allows the user to interface macroscopic samples with microscopic conduits. Here, liquid boundaries formed by hydrodynamic forces underneath the MFP confine a flow of solution that can be used to process large surfaces and objects by scanning across them. This concept is versatile and has been used for protein microarraying, complex gradient-formation, multiphase laminar-flow patterning, erasing, localized staining of cells, contact-free detachment of a single cell, the study of how immune cells function in the presence of infection and how cancer cells migrate, and localized perfusion of tissue slices. Many constraints imposed by the monolithic construction of microfluidic channels can now be circumvented using an MFP, opening up new avenues for microfluidic processing. For more information on the microfluidic probe and its advances and use, please see related videos in Nature Materials, Nature Communications and a featured video in AIP Biomicrofluidics.